sp HYBRIDIZATION OF CARBON
The electronic configuration of carbon in its ground state is:
 |
| Ground state |
The promotion of one of the two 2s electrons to the empty pz orbital gives the excited state.
 |
| Excited state |
This time we follow a different course than that used for sp2
hybridization, in which case one 2s and two 2p orbitals were mixed.
Instead, the 2s electron and just one of the three 2p orbitals are mixed
or hybridized to form two new equivalent orbitals. These two new
orbitals are known as sp orbital because they are formed by interaction
of one s and one p orbital. The other two 2p orbitals (py and pz) are left unhybridized. The electronic configuration of carbon in its sp hybridized state is:
 |
| Hybridized state |
Each sp orbital contains an unpaired electron. The shape of an sp orbital is similar to that of an sp2 orbital.
 |
| Formation of equivalent sp orbitals |
They
sp orbitals obtained are identical, that is, they have same the same
energy and shape. They differ only in their orientation in space with
respect to each other. They lie in a straight line, that is, the angle
between the two sp orbitals is 180°.
 |
| Orientation of two sp orbitals |
The linear arrangement is favoured
because it allows the sp orbitals to stay as far apart from each other
as possible and thereby reducing the electron-electron repulsions. The
unhybridized py and pz orbitals are at right angles to the line of the sp orbitals.
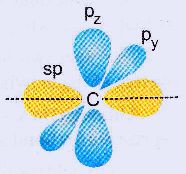 |
| Orientation of the unhybridized orbitals |
Whenever carbon is bonded to two other atoms or groups it always uses sp hybrid orbitals and two 2p (py and pz) orbitals to form its bonds. For example, acetylene.
 |
| Bonding in acetylene |
BONDING IN ACETYLENE
In
acetylene (H-C≡C-H) each carbon atom is attached to one hydrogen atom
by a single covalent bond and to another carbon atom by a triple bond.
Since each carbon is attached to two other atoms it uses sp hybrid
orbitals and two unhybridized 2p orbitals (py and pz) to form its bonds.
In
acetylene there are two C-H single covalent bonds and one C-C triple
bond. Each C-H bond is a sigma bond and results from the overlap of an
sp orbital from carbon and 1s orbital from hydrogen.
One
of the three bonds in C-C triple bond is also a sigma bond and results
from the linear overlap of the two sp orbitals, one from each carbon.
The
other two bonds in the triple bond are pi bonds and results from the
lateral overlap of the unhybridized p orbitals on each carbon.
 |
| Formation of the two pi bonds in acetylene |
Although
the C-C triple bond is represented by three equivalent lines, remember
that one line represents a sigma bond and the other two the pi bonds.
 |
| Triple bond in acetylene |
No comments:
Post a Comment